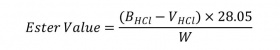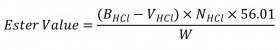Ester value
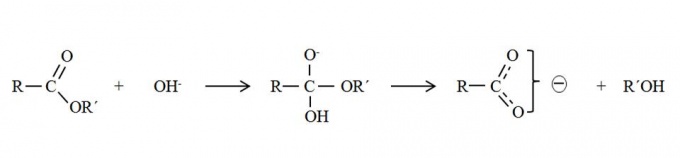
The ester value is the number of mg of potassium hydroxide required to saponify the esters in 1.0 g of the substance.[1]
Contents
Wax esters
Wax esters are oxoesters of long-chain fatty acids esterified with long-chain alcohols. The ester value shows the amount alkali consumed in the saponification of the esters[2] and is possible identify and differentiate the waxes with this value; for example beeswax ester value is 72 to 79 mg KOH/ g, candelilla wax ester value is 31 to 43 mg KOH/g and carnauba wax ester value is 74 to 78 mg KOH/g.
Method
In ester value determination, the sample is hydrolysed to alcohol and using excess of standar potassium hydroxide solution. The excess of alkali is back titrated. USP-NF monographs presents a general procedure of ester value apply to fats, fixed oils and waxes.
USP 401
Place 1.5 g to 2 g of the substance in a tared, 250 mL flasks, add 20 mL to 30 mL of neutralized alcohol and shake. Add 1 mL of phenolphthalein, and titrate with 0.5 N alcoholic potassium hydroxide until the free acid is neutralized. Add 25.0 mL of 0.5N alcoholic potassium hydroxide. Heat the flask on a steam bath, under a suitable condenser to maintain reflux for 30 minutes, frequently rotating the contents titrate the excess potassium hydroxide with 0.5 N hydrochloric acid. Perform a blank determination under the same conditions. Calculate the ester value by the formula:
BHCl: is the volume in mL, of the hydrochloric acid consumed by the blank
VHCl: is the volume in mL, of the hydrochloric acid consumed by the actual test
W: is the weight, in g, of the sample taken
It is possible to perform this test using the sample at the end of determination of acid value, adding 15 mL of potassium hydroxide 0.5 N and heat the flask under a suitable condenser to maintain reflux for 3-4 h. Titrate the excess potassium hydroxide with 0.5 N hydrochloric acid (until the sample turns white). Perform a blank determination under the same conditions. Register the volume of hydrochloric acid consumed for the sample as the well blank. Calculate ester value by the formula:
BHCl: is the volume in mL, of the hydrochloric acid consumed by the blank
VHCl: is the volume in mL, of the hydrochloric acid consumed by the actual test
NHCl: is the normality of the hydrochloric acid
56.1: is the molecular weight of potassium hydroxide
W: is the weight, in g, of the sample taken